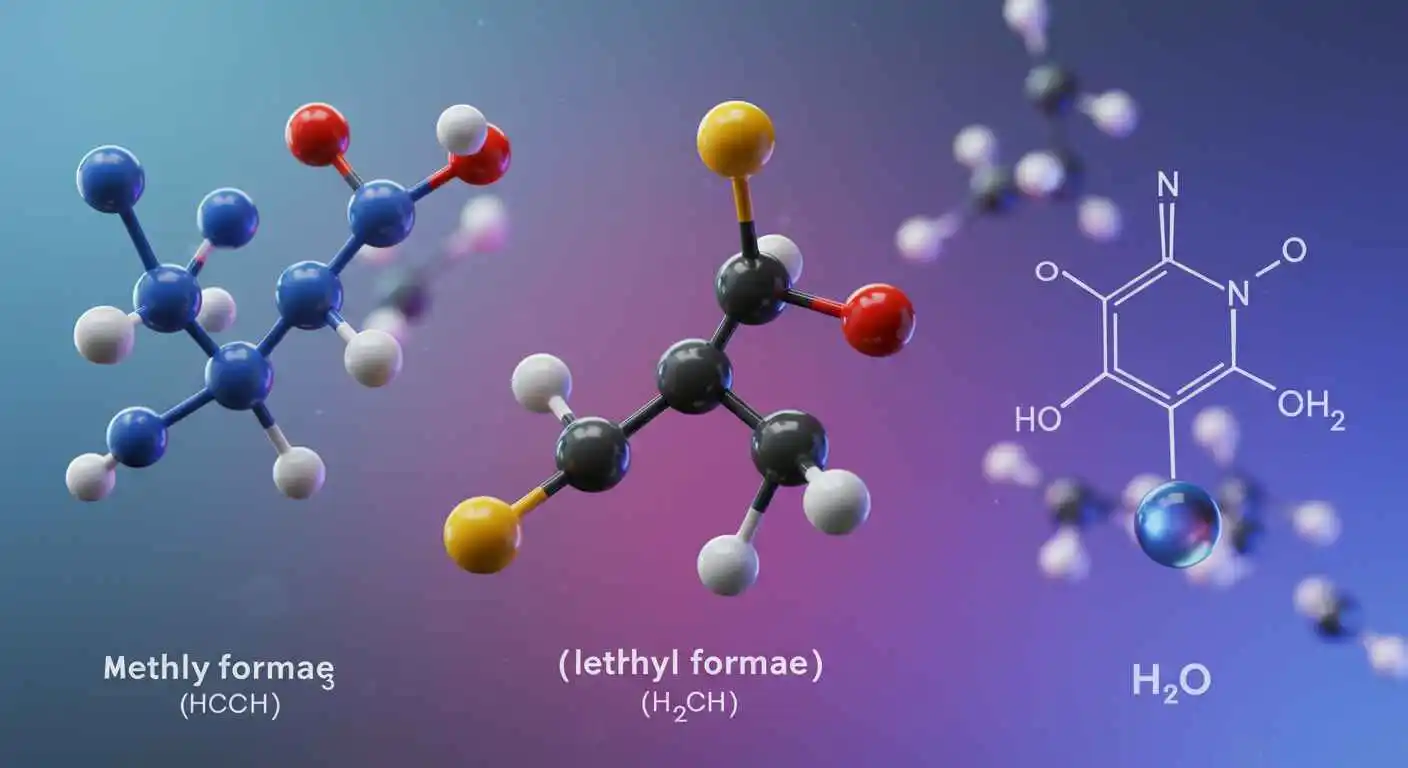
Even the smallest molecules can have significant effects in the fascinating field of chemistry. Examining molecules such as HCOOCH₃ (methyl formate), CH₂ (methylene), and H₂O (water) in detail reveals a fascinating field of organic and aqueous chemistry with significant applications in industry, biology, and environmental science.
The fundamentals of these molecules, their functions, reactions, and the reasons that knowledge of them is important for both science and daily life will all be covered in this article.HCOOCH₃, CH₂, and H₂O
HCOOCH₃ (Methyl Formate): What is it?
The methyl ester of formic acid is called methyl formate. It smells nice and fruity and is a volatile organic substance. Methyl formate’s characteristics make it useful in:
- Fragrances and scents
- Solvents used in industry
- Propellers for aircraft
Methyl formate’s structure can be expressed as H–C=O–O–CH₃, and its chemical formula is HCOOCH₃.
In the production of organic compounds, this molecule serves as a crucial intermediary. It is useful in a variety of chemical processes due to its reactivity and lightweight nature.
Comprehending Methylene, or CH₂
Usually denoted by the symbol CH₂, methylene is a highly reactive carbene. Due to its extreme instability, it is rarely seen in its free form. Nonetheless, it is essential to numerous chemical processes, including:
- Synthesis of polymers
- Radical chain reactions
- Chemistry of organometallics
Methylene intermediates are typically produced momentarily during chemical reactions in laboratory environments. Despite its brief existence, CH₂ is crucial for the synthesis of complex compounds in materials science and pharmaceuticals.
Water’s (H2O) Function in Chemical Reactions HCOOCH₃, CH₂, and H₂O
In addition to being the source of life, water, or H2O, is a potent polar solvent that supports numerous essential chemical reactions.
- Reactions of hydrolysis
- Hydration of organic substances
- serves as a conduit for the chemistry of acids and bases.
Water frequently plays a role in hydrolysis, which breaks down esters like methyl formate (HCOOCH₃) into methanol and formic acid.
The Reaction of Hydrolysis: HCOOCH₃, CH₂, and H₂O
The hydrolysis of methyl formate in water by an acid or base is a well-known and significant reaction:
CH₃OH + HCOOH → HCOOCH₃ + H₂O
This implies:
- Water and methyl formate combine to generate formic acid and methanol.
This response is:
- extensively utilized in labs for organic chemistry
- crucial to the synthesis of pharmaceuticals
- Investigated esters’ environmental biodegradation
It is an excellent illustration of green chemistry in action because it is safe, effective, and eco-friendly.
What Role Does CH₂ Play?
Chemists involve CH₂ in sophisticated synthetic processes, even though it does not directly participate in the hydrolysis of HCOOCH₃.
- The process of cyclopropanation
- Olefination
- Reactions of insertion
Methylene frequently occurs as a precursor or intermediate in multi-step processes involving esters, such as methyl formate.
Applications of HCOOCH₃, CH₂, and H₂O in the Fragrance and Flavor Industry in the Real World: Because of its pleasant, fruity aroma, methyl formate is widely used.
-
Scientists have even found methyl formate in interstellar clouds, indicating the presence of organic chemistry in space beyond Earth.
-
Researchers are investigating esters such as HCOOCH₃ for applications in bio-based fuels and solvents.
- Synthetic Pathways: Scientists can create biodegradable items by knowing how water breaks down esters.
Why This Chemistry Is Important for the Environment:
- Water-based processes, such as the hydrolysis of methyl formate, reduce reliance on hazardous solvents.
- Educational Value: Teaching organic reaction mechanisms requires an understanding of these reactions.
- Astrochemical Significance: Methyl formate’s discovery in space raises interest in the study of the cosmos.
- Environmentally aware chemists prefer these gentle and effective reactions for green synthesis..
Tips for Safety and Handling
- Methyl Formate is flammable and somewhat poisonous. Use in well-ventilated locations at all times.
- Chemists typically produce extremely reactive CH₂ intermediates only in carefully monitored laboratory environments.
- Water is safe and necessary for chemistry; however, for the greatest reaction outcomes, use pure, distilled, or deionized water.
The Simplicity’s Scientific Basis
The hydrolysis of HCOOCH₃, albeit a straightforward reaction, highlights significant chemical ideas like:
- Attack by electrophilia
- Substitution by nucleophiles
- Energy diagrams and transition states
This makes it an excellent starting point for chemists and students studying chemical equilibrium and reaction kinetics.
How to Hydrolyze Methyl Formate: A Comprehensive Guide (For Educational Purposes Only)
- Make a diluted base or acid catalyst, such as sodium hydroxide or diluted hydrochloric acid.
- In a reaction vessel, combine water and methyl formate at a regulated temperature.
- To start the hydrolysis, add the catalyst gradually while stirring constantly.
- Track the development of the reaction by looking for the production of methanol and formic acid.
- Once finished, carefully separate the products and purify if required.
Professionals should carry out this reaction in a properly equipped lab environment using appropriate safety gear.
Commonly Asked Questions
What occurs when H₂O and HCOOCH₃ react?
It is hydrolyzed, yielding methanol and formic acid.
Is the free form of CH₂ stable?
No. Due to its high reactivity, methylene mostly exists as a temporary intermediate.
Where in the real world is methyl formate used?
in propellants, solvents, fragrances, and even space research.
Is it environmentally friendly to hydrolyze methyl formate?
Indeed. Because of its gentle conditions and use of water as a solvent, it is an excellent illustration of green chemistry.
Is it possible to perform this reaction at home?
No. Methyl formate should only be handled by qualified personnel due to safety concerns.
What role does CH₂ play in synthetic chemistry?
In materials science and pharmaceuticals, it creates extremely reactive intermediates that are essential for creating complex compounds.
In conclusion
The trio of HCOOCH₃, CH₂, and H₂O is a fascinating intersection of aqueous, organic, and even astrochemistry. These molecules demonstrate how basic chemistry may result in long-lasting, useful, and cosmic insights, from lab experiments to the chemistry found in far-off stars.
Knowing these substances not only improves our comprehension of the microscopic world but also stimulates advancements in environmental science, industry, and other fields.